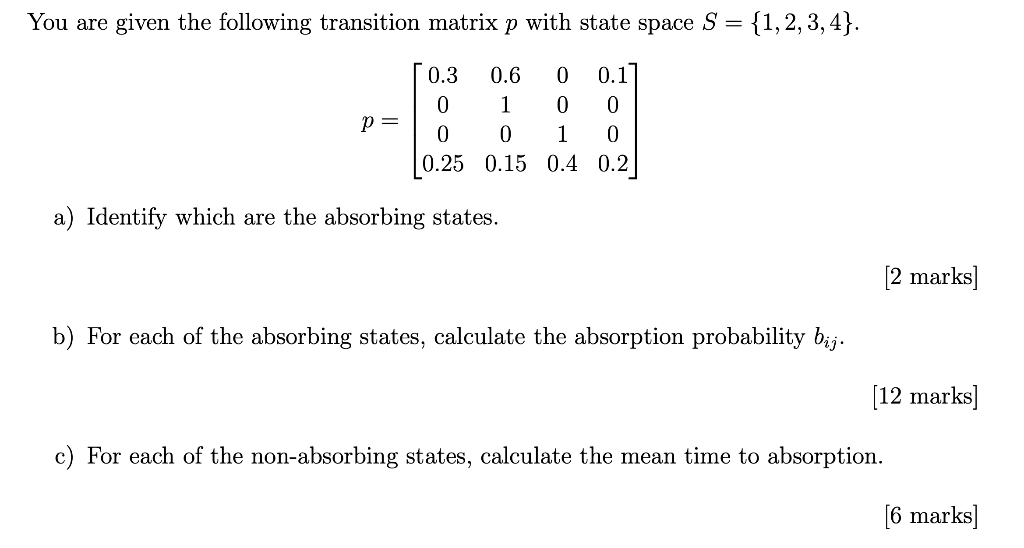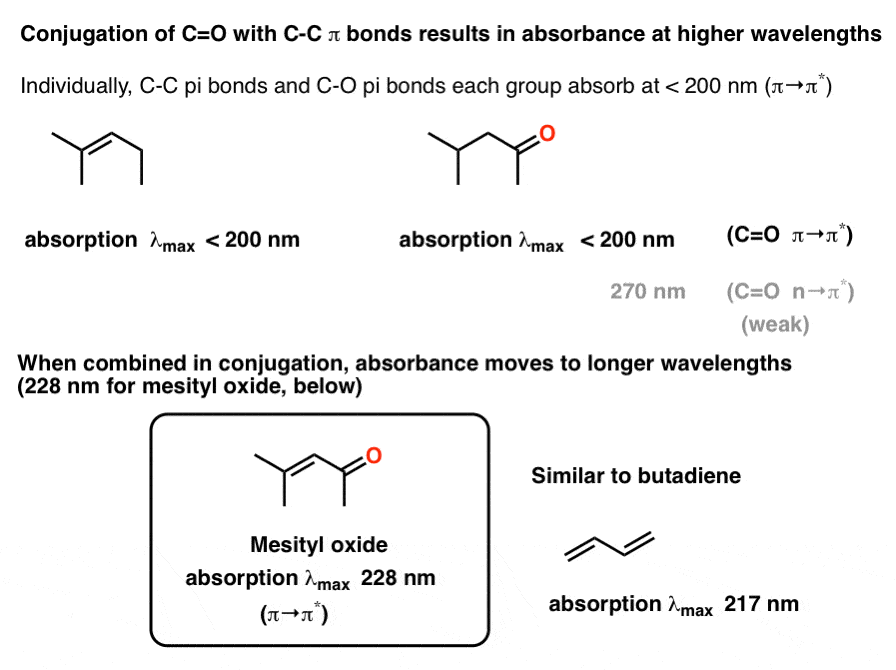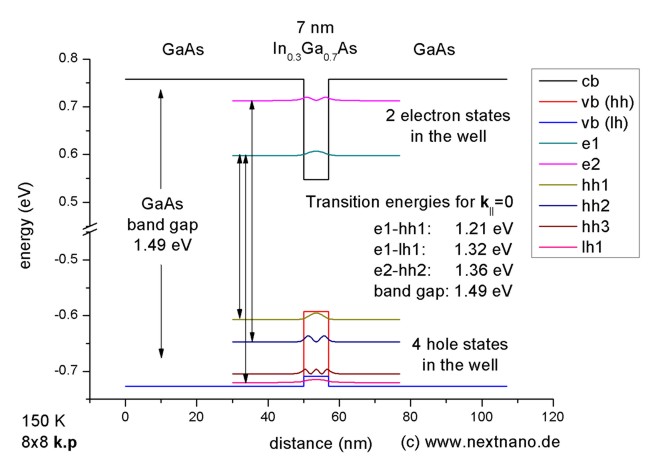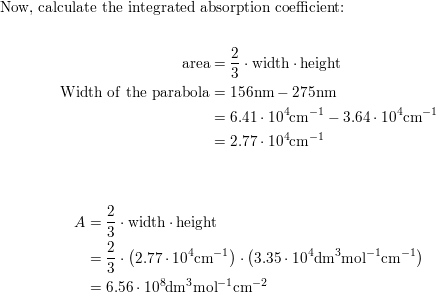
The longest wavelength doublet absorption transition is observed at 589.0 ans 589.6nm . Calculate the frequency of each transition and energy difference between two excited states .

In a one-body picture, there is only one transition in the absorption... | Download Scientific Diagram
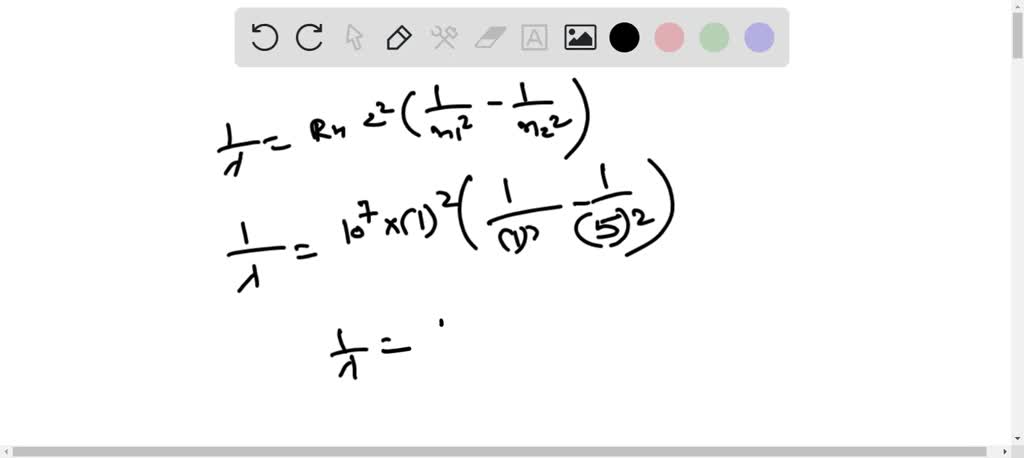
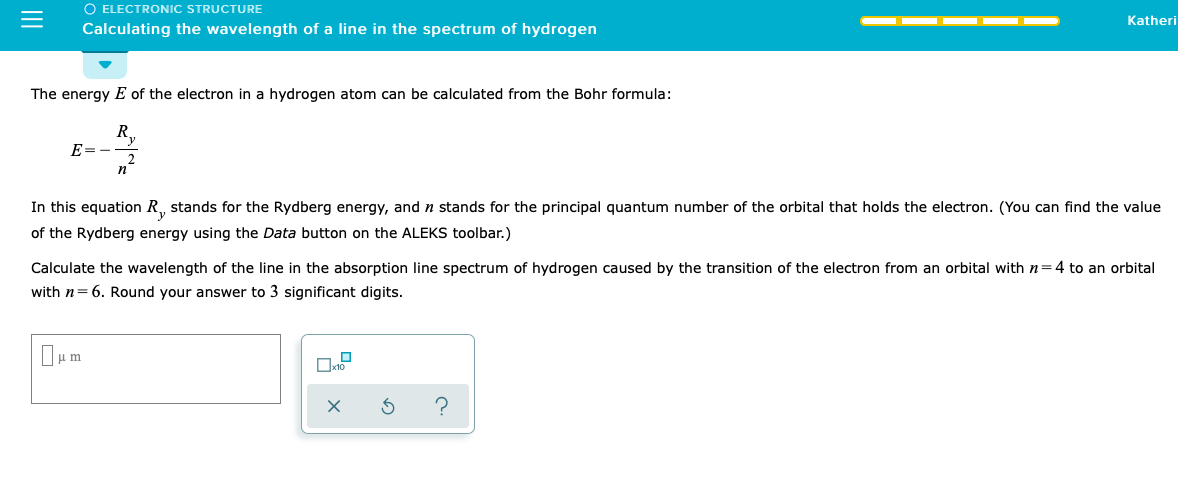
SOLVED: Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=1 to an orbital with n=5. Round
CALCULATION OF THE TRANSITION DIPOLE MOMENT OF THE $\tilde{A}\leftarrow \tilde{X}$ ELECTRONIC TRANSITION OF THE C$_2$H$_5$O$_2$ FROM THE PEAK ABSORPTION CROSS-SECTION
CALCULATION OF THE TRANSITION DIPOLE MOMENT OF THE $\tilde{A}\leftarrow \tilde{X}$ ELECTRONIC TRANSITION OF THE C$_2$H$_5$O$_2$ FROM THE PEAK ABSORPTION CROSS-SECTION

Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the
CALCULATION OF THE TRANSITION DIPOLE MOMENT OF THE ˜A ← ˜X ELECTRONIC TRANSITION OF THE C2H5O2 FROM THE PEAK ABSORPTION CROS

The longest wavelength doublet absorption transition is observed at 589 and 589.6 nm. Calculate the frequency of each transition and energy difference between two excited states.
![PDF] Calculation of single-beam two-photon absorption transition rate of rare-earth ions using effective operator and diagrammatic representation | Semantic Scholar PDF] Calculation of single-beam two-photon absorption transition rate of rare-earth ions using effective operator and diagrammatic representation | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0045c9a6c975d5467e09bdc57a57de9752d40b5/8-Figure1-1.png)
PDF] Calculation of single-beam two-photon absorption transition rate of rare-earth ions using effective operator and diagrammatic representation | Semantic Scholar

CHEM1011 notes for 8 topics - Atomic spectra & Rydberg 1. Relate the lines and series in the - Studocu

SOLVED: 2) Which of these transitions correspond to emission and which to absorption? a. n = 2 to n = 4, Absorption b n = 3to n = 1, Emission C. n =
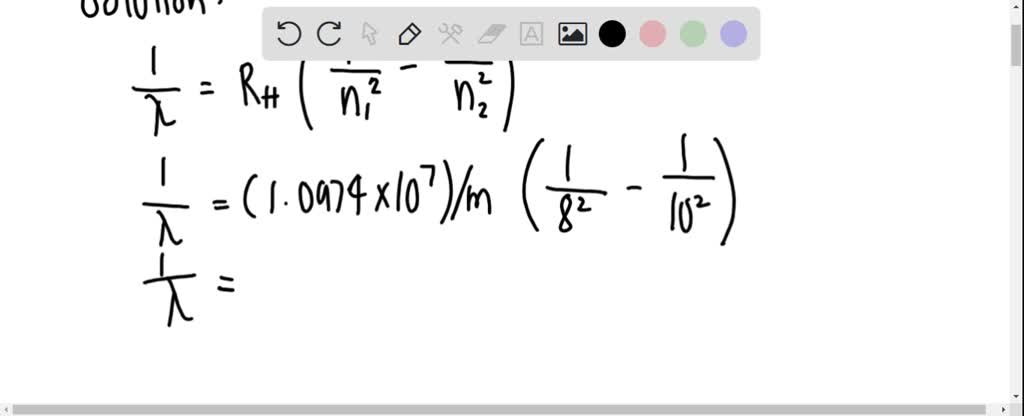
SOLVED: Calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=8 to an orbital with n=10. Round

Femtosecond Absorption Spectroscopy of Transition Metal Charge-Transfer Complexes | Accounts of Chemical Research