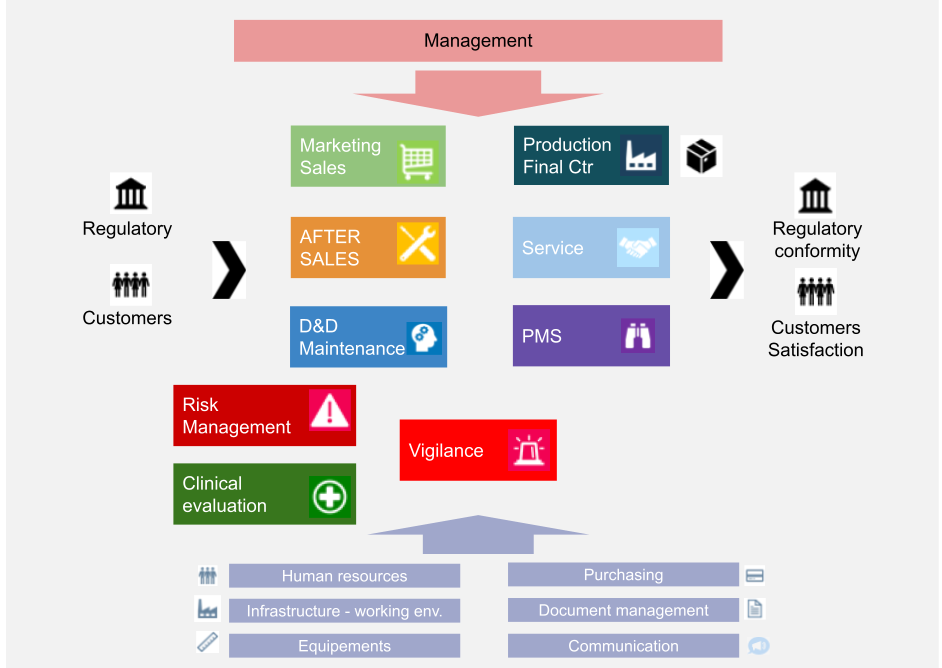
Lead Auditor Training & Certification on MD-QMS Requirements for Regulatory Purposes based on ISO 13485 | TÜV SÜD in India
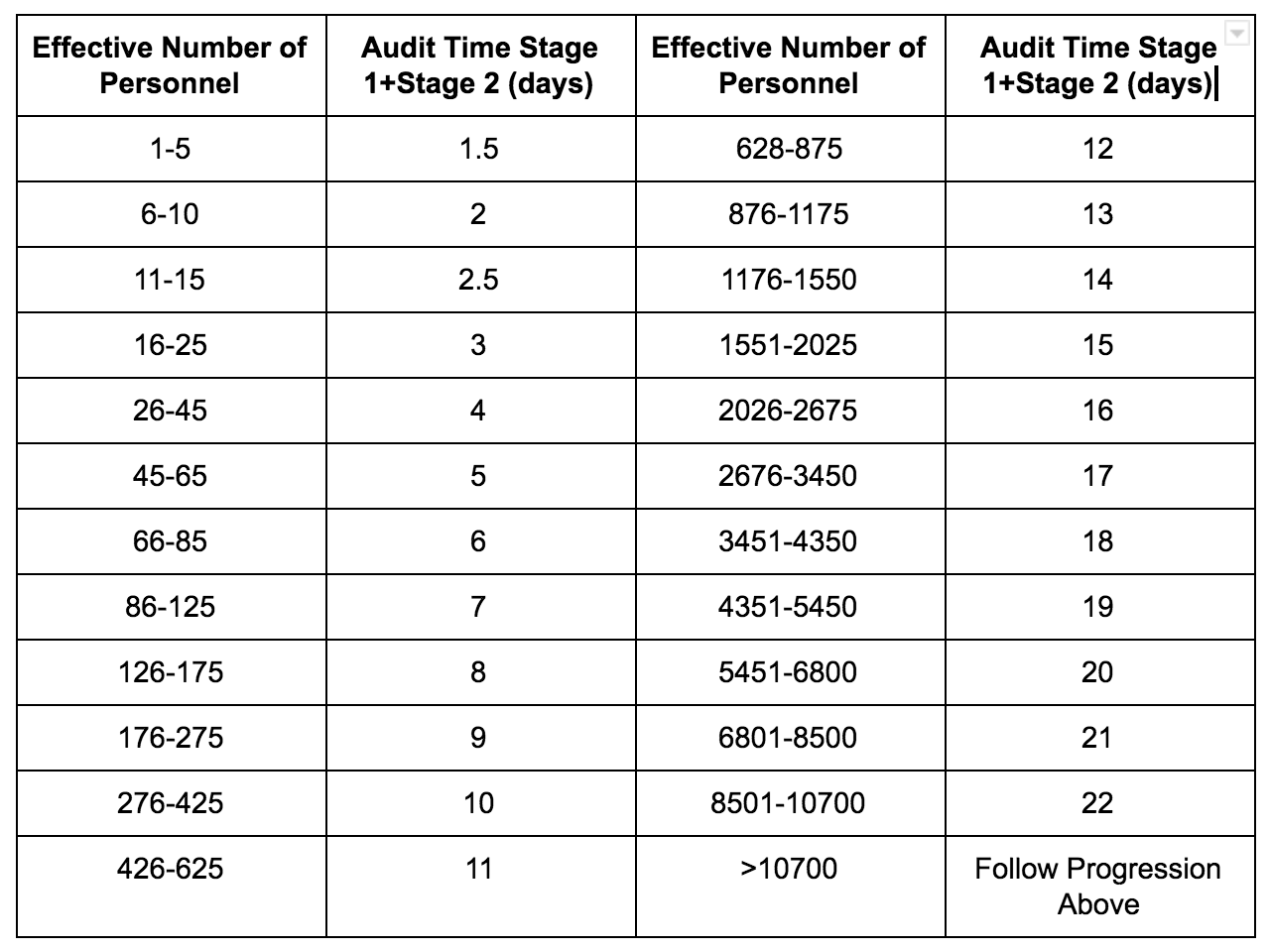
IAF Mandatory Document Determination of Audit Time of Quality and Environmental Management Systems (IAF MD 5: 201X)
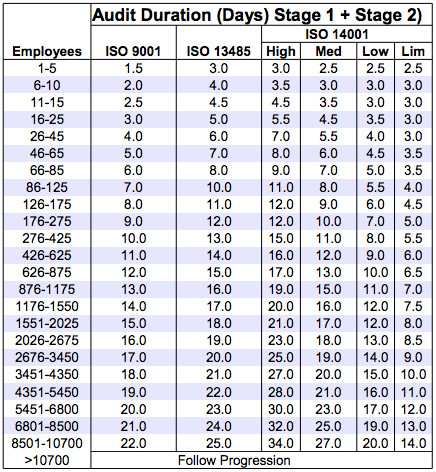
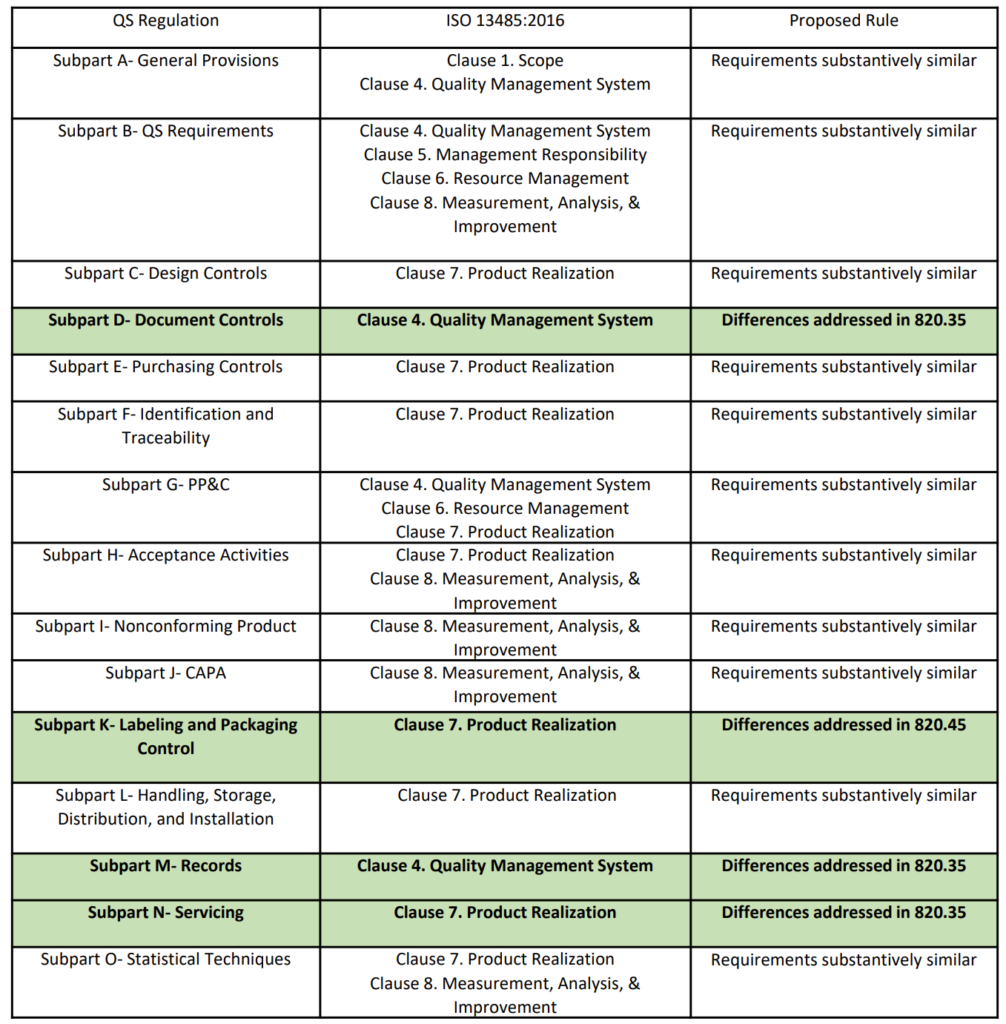
IAF Mandatory Document Application of ISO/IEC 17021-1 in the Field of Medical Device Quality Management Systems (ISO 13485) Issu


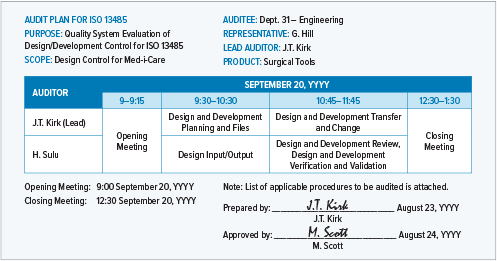
.png?width=250&height=324&name=UG%20ISO%2013485%20(1).png)